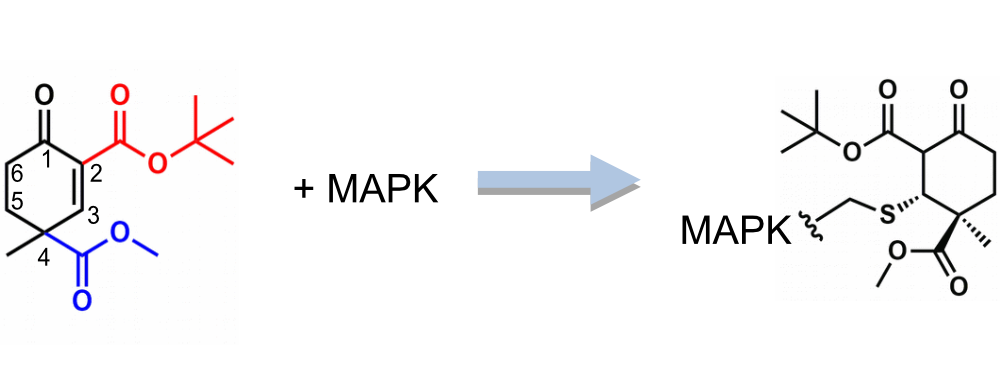
Project examples
The following two examples demonstrate the unique features and capability of Protenic’s cyclopentenone/cyclohexenone based warheads.
MAPK D-groove
Mitogen-activated protein kinases (MAPK) have a shallow protein-protein interaction surface, the so-called docking groove (D-groove). We developed compounds that form a reversible covalent bond with a universally conserved cysteine located in the middle of the D-groove. These compounds compete with the binding of MAPK partner proteins and thus interfere with MAPK signaling involved in cancer and inflammatory diseases.
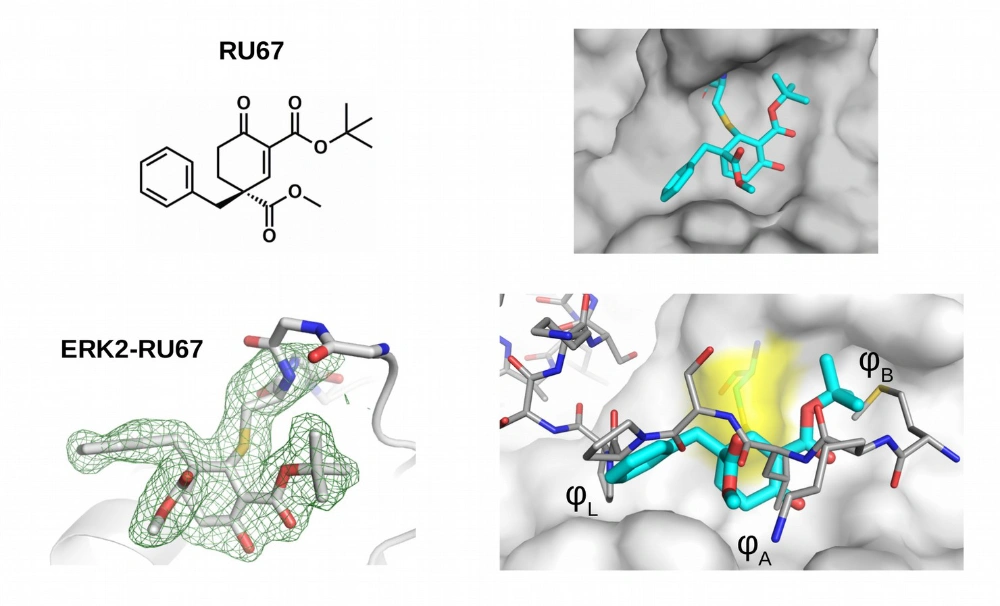
RU67 (in cyan in the right panels) binds to the MAPK D-groove cysteine (C161, in yellow, located in the central position of a shallow surface area comprised of small hydrophobic pockets; φL,A,B) and blocks peptide binding (right bottom panel show the overlay of a MAPK–D-peptide, in gray, and the MAPK-RU67 crystal structures).
Depending on the substituent groups and/or the configuration at C4, cyclohexenone based compounds may bind to the MAPK D-groove cysteine or nearby histidine (C161 or H125 in ERK2).
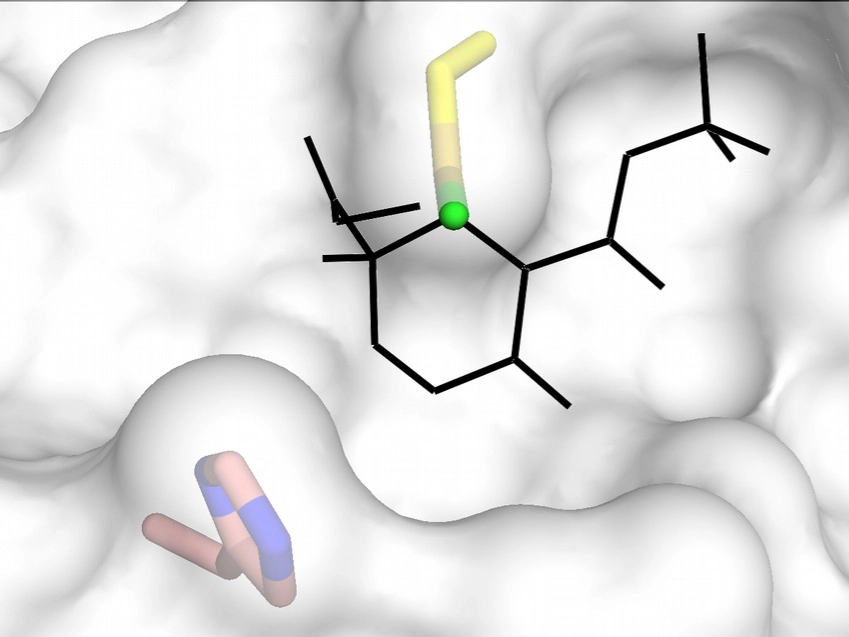
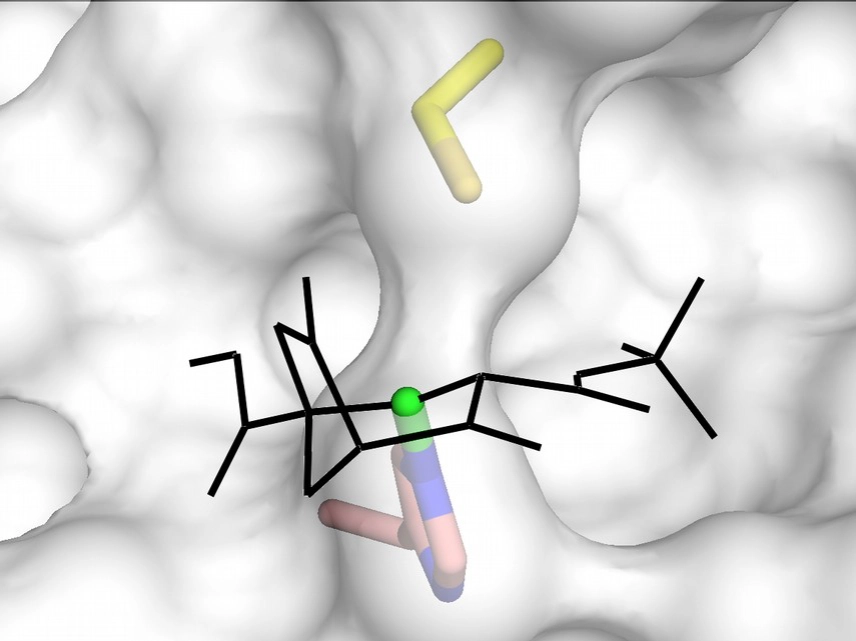
For more information please see:
https://www.nature.com/articles/s41467-024-52574-1
JNK ATP-competitive inhibitors
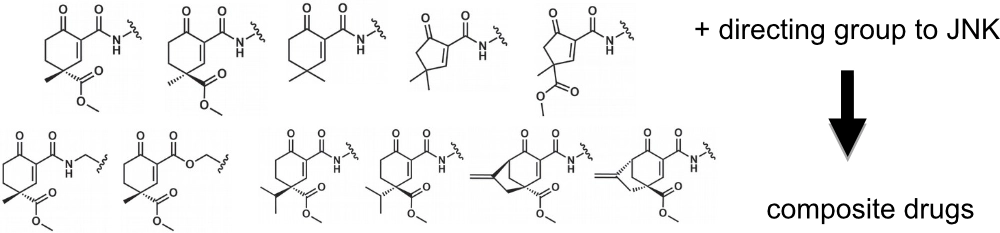
Different cyclopentenone/cyclohexenone based warheads can be added to known directing groups (DG; e.g., a noncovalent moiety known to bind to the ATP-pocket of JNK) and the latter will “guide” the warhead to specific proteins and to specific sites. A reversible covalent bond to a nearby nucleophile (cysteine or histidine) will increase the binding affinity and more importantly will increase residence time.
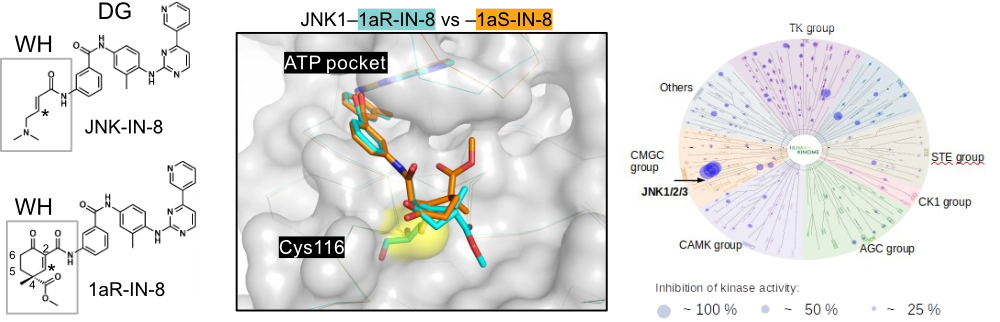
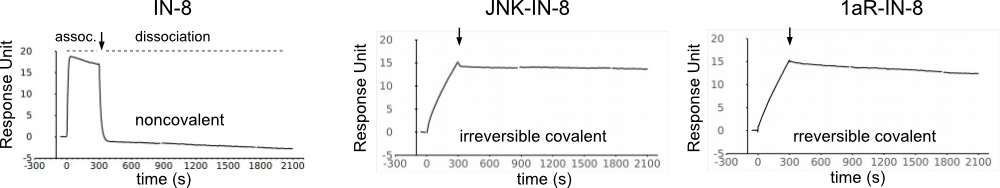
The new composite JNK inhibitors have a cyclic warhead with a stereogenic center at C4. This allows the positioning of the substituent groups differently which is important to achieve great JNK isoform selectivity.
For more information please see:
https://www.nature.com/articles/s41467-024-52573-2